https://files.peakd.com/file/peakd-hive/agmoore/23viTPVmXrBxbTYiCMKkyuFtTLhc443nUGqmog4LCpm2rScFARxbhD7FfLjbhLw3Kizon.jpg
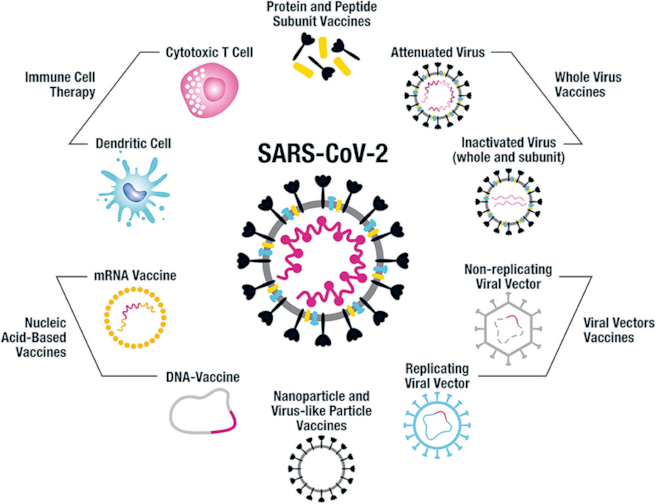
*Image credit: Katie L. Flanagan, Emma Best, Nigel W. Crawford, Michelle Giles, Archana Koirala, Kristine Macartney, Fiona Russell, Benjamin W. Teh, and Sophie CH Wen, on behalf of the Australasian Society for Infectious Diseases (ASID) Vaccination Special Interest group (VACSIG). Used under a [CC 4.0 license.](https://commons.wikimedia.org/wiki/File:Fimmu-11-579250-g004.jpg) The caption under this picture [states:](https://www.frontiersin.org/articles/10.3389/fimmu.2020.579250/full) "This figure illustrates the different vaccine approaches being taken for the design of human SARS-CoV-2 vaccines".*

Different countries have different protocols for approving drugs for use in the general population. Sometimes approval comes with caveats for specific segments of the population. Some of the caveats that concern people with autoimmune diseases may be under appreciated. This is true of both therapeutics (they treat diseases) and vaccines (they prevent diseases). I have been diagnosed with a couple of autoimmune diseases and have found that doctors either are not aware of the special concerns, or do not share these concerns with me when making recommendations, especially recommendations for vaccines. So, in this blog I will go through some of the recent literature about the safety/efficacy of various vaccines, and vaccine types. ***Antibodies Binding to DNA in Systemic Lupus, an Autoimmune Disease***
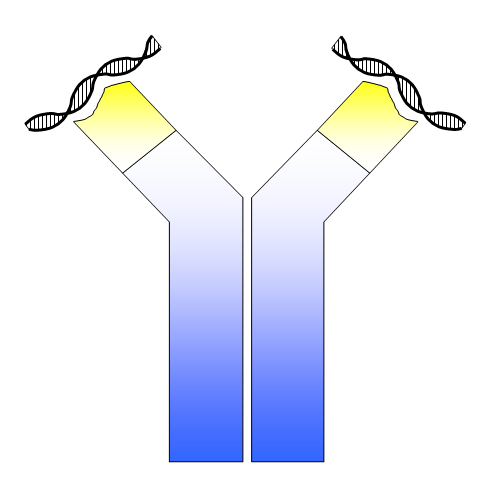
*Image credit: Simon Caulton. Used under a [CC 3.0 license.](https://commons.wikimedia.org/wiki/File:ANA_-_dsDNA_antibody.png) The caption under this illustration reads: "Antibody binding to DNA." It is this antibody binding that is the hallmark of autoimmune diseases. The presence of autoantibodies causes inflammation, which leads to symptoms of an autoimmune disease.* ###
Stricter Rules for Vaccines as a Class of Drugs
***Polk State School For the Feeble Minded Where Jonas Salk Tested the Polio Vaccine on Children***

*Image credit: National Park Service. [Public domain.](https://commons.wikimedia.org/wiki/File:POLK_STATE_SCHOOL_AND_HOSPITAL._-_Polk_State_School_and_Hospital,_Main_Street_at_School_Drive,_Polk,_Venango_County,_PA_HABS_PA-6746-6.tif) The disabled children at this school [received the vaccines in 1952,](https://studylib.net/doc/7614153/transcript-of-ann-r.-keefer-s-lecture--segment-of-a-lecture) two years before the full field test had taken place.* The recent pause in administering two COVID 19 vaccines, [AstroZenica](https://www.wsj.com/articles/oxford-pauses-dosing-in-trial-of-astrazeneca-covid-19-vaccine-in-children-teenagers-11617729303) and [J&J,](https://www.cnn.com/2021/04/13/health/johnson-vaccine-pause-cdc-fda/index.html) highlights the strict safety standards to which vaccines are held today. These standards are more stringent than those applied to therapeutic drugs. This is because, as a statement by the United States Centers for Disease Control [explains:](https://www.cdc.gov/vaccines/pubs/pinkbook/safety.html) >A higher standard of safety is generally expected of vaccines than of other medical interventions because, in contrast to most pharmaceutical products that are administered to ill persons for treatment purposes, vaccines are generally administered to healthy persons to prevent disease. In other words, in the effort to keep healthy people from becoming ill, pharmaceutical should not make them sick. However, the history of vaccines reveals less careful, even cavalier, vetting processes. ***Edward Jenner Vaccinating James Phipps, May 4, 1796***
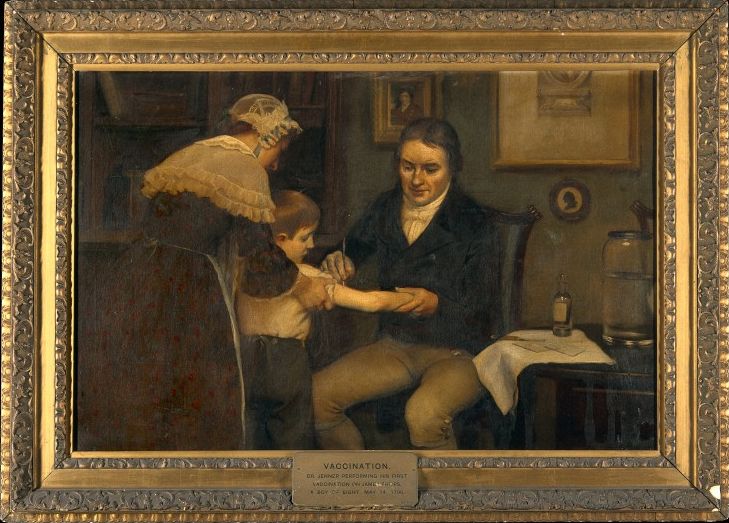
*Image credit: Ernest Board (1877-1934);Images.wellcome.ac.u. [Public domain.](https://commons.wikimedia.org/wiki/File:Jenner_phipps_01.jpg) In this famous scene, Jenner is vaccinating an 8-year-old boy in the hopes that the vaccine will confer immunity to smallpox. Although this incident was heralded as a breakthrough in smallpox prevention, [the ethics](https://dash.harvard.edu/bitstream/handle/1/37945151/DELEON-DOCUMENT-2018.pdf?sequence=1&isAllowed=y) of this experimental use of the vaccine have been challenged.* The urgency that led to Jenner's audacious use of the smallpox vaccine may be understood in terms of the disease's lethality at the time, and its potential to disable. It is estimated that in 18th-century London, death rates from smallpox ranged from between [10 to 30%](https://www.sciencedirect.com/science/article/pii/S0277953618301862) of total deaths. Those it did not kill, it often disfigured. And among those disfigured were many who were [rendered blind by the disease.](https://jamanetwork.com/journals/jamaophthalmology/fullarticle/415346) Today, the world is confronted with a pandemic of COVID-19 that has spread virtually unchecked for at least a year. The virus has mutated dramatically in that year. Our sense of urgency helps us to understand Jenner's urgency. The [highly accelerated rate](https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-in-2021) with which COVID-19 vaccines have been approved has happened in the context of urgency. ***New York Javits Center Converted to a Hospital, April 3, 2020***

*Image credit: New York National Guard. Used under [CC 2.0 license.](https://commons.wikimedia.org/wiki/File:New_York_National_Guard_-_49738408268.jpg) New York became an epicenter of the pandemic in March and April of 2020. According to Wikipedia, there were [29,000](https://en.wikipedia.org/wiki/COVID-19_pandemic_in_New_York_(state))* excess deaths in New York that month (over the death toll of April 2019). However, the accelerated approval process has increased vaccine hesitancy in some populations. I personally have been hesitant, not because the vaccine itself is worrisome (well, yes, of course I have reasonable concerns) but because I am part of a sub-group for whom vaccines may present particular issues: people with an autoimmune inflammatory rheumatic disease, hereafter referred to as AIIRD. I have never had a discussion about these particular vaccine issues with any of my physicians. I'm not sure if this reflects their reluctance to discuss the issues, or the fact that they don't understand the issues. However, peer reviewed literature on the subject is clear, and I will try to summarize some of that here.

***SARS-CoV-2 Emerging From the Surface of Cells, Electron Microscope Scan***
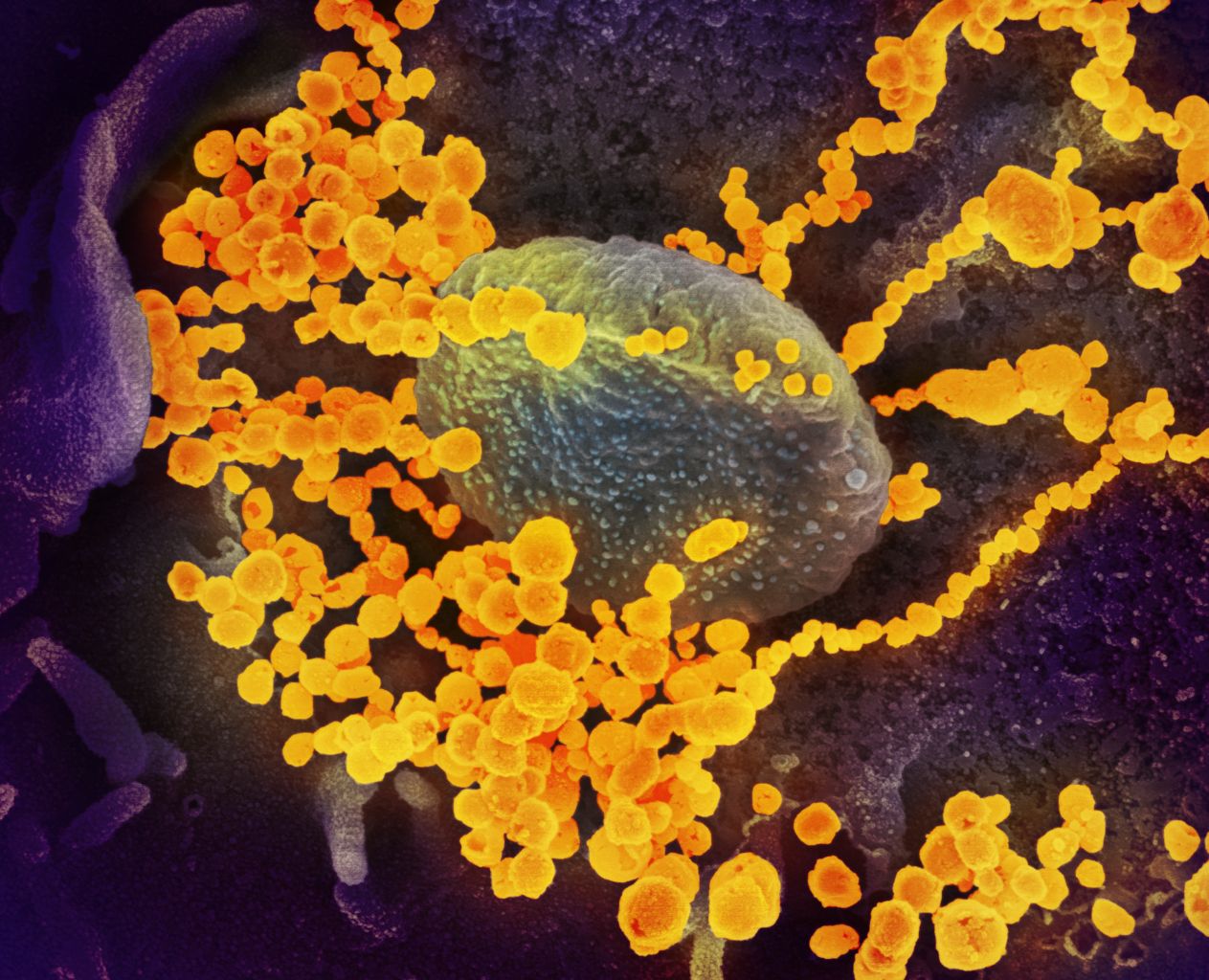
*Image credit: National Institute of Allergy and Infectious Diseases (NIAID). Used under a [CC 2.0 license](https://commons.wikimedia.org/wiki/File:SARS-CoV-2_scanning_electron_microscope_image.jpg)* ###
COVID-19 Vaccinations
If readers of this blog have been following even casually the discussion about COVID-19 vaccines, they understand that the goal of the vaccine is to produce antibodies against the virus. But wait! People with autoimmune diseases already produce *too many antibodies*. Isn't that a problem? Well, yes, maybe. The difficulty with answering this question is, the vaccines haven't been around long enough to know for sure what will happen to antibody production in a person with an autoimmune disease. On March 24, 2021, the [Boston Medical Journal published](https://ard.bmj.com/content/early/2021/03/24/annrheumdis-2021-220231) a safety review of COVID-19 vaccines for people with autoimmune diseases. There are a couple of concerning statements in that article. One of the statements is that people with autoimmune diseases were deliberately excluded from the trials. Another is that 'adverse events' reported in the general populations may be more severe in "patients with underlying immune dysregulation". ***Sniffer Dog Marking A Cone in COVID-19 Detection Test***

*The results of this dog sniffer test were published in [Plos One.](https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0243122) The study demonstrated that trained dogs could detect COVID-19 in the sweat of affected patients. The results need to be repeated in order to be useful. ***Image credit***: Dominique Grandjean, Riad Sarkis, Clothilde Lecoq-Julien, Aymeric Benard, Vinciane Roger, Eric Levesque,Eric Bernes-Luciani,Bruno Maestracci, Pascal Morvan, Eric Gully, David Berceau-Falancourt, Pierre Haufstater, Gregory Herin, Joaquin Cabrera, Quentin Muzzin, Capucine Gallet, Hélène Bacqué, Jean-Marie Broc, Leo Thomas, Anthony Lichaa, Georges Moujaes, Michele Saliba, Aurore Kuhn, Mathilde Galey, Benoit Berthail, Lucien Lapeyre, Anthoni Capelli, Steevens Renault,Karim Bachir, Anthony Kovinger, Eric Comas, Aymeric Stainmesse, Erwan Etienne, Sébastien Voeltzel, Sofiane Mansouri, Marlène Berceau-Falancourt, Aimé Dami,Lary Charlet, Eric Ruau, Mario Issa, Carine Grenet, Christophe Billy, Jean-Pierre Tourtier, Loïc Desquilbet. Used under a [CC 4.0 license](https://commons.wikimedia.org/wiki/File:A_sniffer_dog_marking_a_cone_on_a_4-cone_lineup.png)*

A [report](https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf) by the American College of Rheumatology suggests the possibility that the vaccine may also precipitate a 'flare' (exacerbation of disease) in AIIRD patients. In addition to the theoretical risks to AIIRD patients, there is the added concern of reduced immunogenicity. That is, the vaccine may not produce enough antibodies in this population to offer protection against COVID-19. This may be the case because people who have an autoimmune condition are likely to be taking immune suppressive drugs. These drugs [may suppress]((https://www.reuters.com/article/us-health-coronavirus-science/autoimmune-disease-drugs-may-reduce-vaccine-response-antibody-treatments-ineffective-vs-brazil-variant-idUSKBN2BZ2H1) ) the hoped-for antibody production of a COVID-19 vaccine. So, are the vaccines suitable for people who have autoimmune diseases? Keep in mind as you consider this question that there are whole classes of autoimmune diseases that are separate from rheumatology. The same ACR report that described potential difficulties with COVID-19 vaccines also states that, "AIIRD patients are at higher risk for hospitalized COVID-19 and worse outcomes compared to the general population." And, "AIIRD patients should be prioritized for vaccination before the non-prioritized general population of similar age and sex." The bottom line,therefore is (for me), AIIRD patients are probably at a greater risk for severe/fatal COVID-19. Vaccines are not an ideal solution. However, at the moment, they are the only solution. So, in accordance with the ACR recommendation I am getting a vaccine. The ACR hastens to add: "ACR guidance statements are not intended to supersede the judgement of rheumatology care providers *nor override the values and perspectives of their patients*". The vaccine offers not only possible immunity. It also is a gift to my family, who otherwise would be hostage to my COVID-19 susceptibility. ###
Other Types of Vaccines For AIIRD Patients
*Attenuated Live Virus and Killed Virus*
***World Tetanus Deaths by Age Group, 1990-2017***
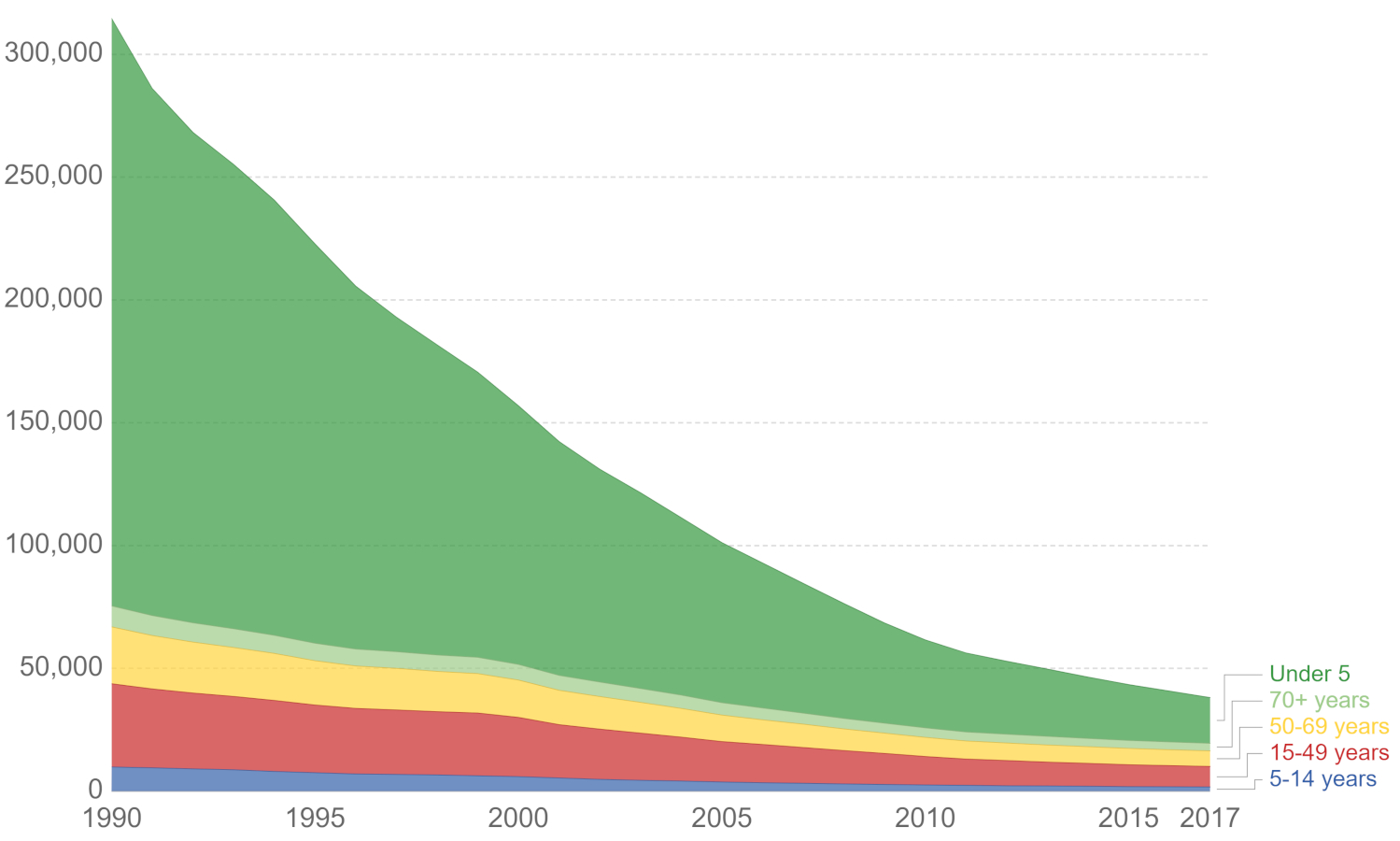
*Image credit: Our world Data. Used under a [CC 4.0 license.](https://commons.wikimedia.org/wiki/File:Tetanus-deaths-by-age-group.png)* In May of 2020, EULAR (European Alliance of Associations for Rheumatology) issued [guidance](https://www.eular.org/index.cfm) on vaccinations for people with AIIRD. The guidance suggests that a full assessment of vaccine history, disease status and medication profile be established before a vaccine is administered. One concern is that different medications interact with drugs in different ways. The EULAR guidance includes the recommendation that, if possible, vaccines should be given when the disease is quiescent, and before starting immunosuppressive treatment. Vaccines containing live, attenuated virus are considered separately from those containing killed virus. Generally, people who are taking immunosuppressive drugs should avoid vaccines containing live virus. For example, the Sabin oral polio vaccine contains a live attenuated virus and the Salk vaccine (injection) contains a killed virus. It is actually possible for someone who receives the Sabin vaccine [to give polio](https://www.cdc.gov/vaccines/vpd/polio/hcp/vaccine-derived-poliovirus-faq.html) to a person who is on immunosuppressive drugs. ***Preparation of Sabin Oral Polio Vaccine, Bonn, Germany, 1967***

*[Attribution: Bundesarchiv, B 145 Bild-F025952-0015 / Gathmann, Jens / CC-BY-SA 3.0](https://commons.wikimedia.org/wiki/File:Bundesarchiv_B_145_Bild-F025952-0015,_Bonn,_Gesundheitsamt,_Schutzimpfung.jpg)* In particular, the EULAR guidance on vaccines recommends vaccination occur before treatment with one drug, [Rituximab,](http://chemocare.com/chemotherapy/drug-info/Rituximab.aspx) begins. Live attenuated virus-containing vaccines, such as the MMR and the herpes zoster (shingles), should be avoided or given with extreme caution in people on immune suppressive therapy. According to the EULAR guidance (subject to decisions by doctors and patients), vaccines which contain killed viruses, such as those that target flu, tetanus and hepatitis, can be given without regard to immune suppressive therapy. For a complete description of the EULAR recommendations, please refer to the the [website.](https://www.eular.org/index.cfm) ***Conclusion*** I hope this blog is useful to people who are in the process of getting, or planning to get a COVID-19 vaccine. More than that, I hope people will use the other information about vaccines to make informed choices about their own healthcare. Obviously, I make no recommendations here about a medical decision anyone should make, except for this: be informed. Tomorrow I get my second, booster, Pfizer dose. I made the decision, because of my complicated medical history, to receive the shot at an annex of a hospital I trust. If anything goes wrong, I'm in the best place to get the best care.






1.https://www.frontiersin.org/articles/10.3389/fimmu.2020.579250/full 2.https://studylib.net/doc/7614153/transcript-of-ann-r.-keefer-s-lecture--segment-of-a-lecture 3.https://www.wsj.com/articles/oxford-pauses-dosing-in-trial-of-astrazeneca-covid-19-vaccine-in-children-teenagers-11617729303 4.https://www.cnn.com/2021/04/13/health/johnson-vaccine-pause-cdc-fda/index.html 5.https://www.cdc.gov/vaccines/pubs/pinkbook/safety.html 6.https://dash.harvard.edu/bitstream/handle/1/37945151/DELEON-DOCUMENT-2018.pdf?sequence=1&isAllowed=y 7.https://www.sciencedirect.com/science/article/pii/S0277953618301862 8.https://jamanetwork.com/journals/jamaophthalmology/fullarticle/415346 9.https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-in-2021 10.https://en.wikipedia.org/wiki/COVID-19_pandemic_in_New_York_(state) 11.https://ard.bmj.com/content/early/2021/03/24/annrheumdis-2021-220231 12.https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0243122 13.https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf 14.https://www.reuters.com/article/us-health-coronavirus-science/autoimmune-disease-drugs-may-reduce-vaccine-response-antibody-treatments-ineffective-vs-brazil-variant-idUSKBN2BZ2H1 15.https://www.eular.org/index.cfm 16.https://www.cdc.gov/vaccines/vpd/polio/hcp/vaccine-derived-poliovirus-faq.html 17.http://chemocare.com/chemotherapy/drug-info/Rituximab.aspx 18.https://www.virology.ws/2015/09/10/why-do-we-still-use-sabin-poliovirus-vaccine/ 19.https://digital.sciencehistory.org/works/2227mq74m 20.https://www.sciencedirect.com/science/article/pii/S1074761306003955 21.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7842169/ 22.https://www.wesa.fm/science-health-tech/2018-04-02/site-where-polio-vaccine-was-first-tested-on-humans-to-receive-state-historic-marker

Originally posted here: https://hive.blog/hive-196387/@agmoore/do-people-with-autoimmune-disease-have-special-issues-when-getting-a-vaccine




































No comments:
Post a Comment