https://files.steempeak.com/file/steempeak/darkflame/U8G3PRrK-image.png
"Recent guidelines from South Korea and China report that chloroquine is an effective antiviral therapeutic treatment against Coronavirus Disease 2019. Use of chloroquine (tablets) is showing favorable outcomes in humans infected with Coronavirus including faster time to recovery and shorter hospital stay. US CDC research shows that chloroquine also has strong potential as a prophylactic (preventative) measure against coronavirus in the lab, while we wait for a vaccine to be developed. Chloroquine is an inexpensive, globally available drug that has been in widespread human use since 1945 against malaria, autoimmune and various other conditions." https://docs.google.com/document/d/e/2PACX-1vTi-g18ftNZUMRAj2SwRPodtscFio7bJ7GdNgbJAGbdfF67WuRJB3ZsidgpidB2eocFHAVjIL-7deJ7/pub
Radio show discusses this https://omny.fm/shows/the-christian-outlook-topics-for-todays-believer-1/the-cure-for-covid-19-has-been-found-kevin-mccullo "Chloroquine was discovered in 1934 by Hans Andersag. It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system. It is available as a generic medication. The wholesale cost in the developing world is about US$0.04. In the United States, it costs about US$5.30 per dose." https://en.wikipedia.org/wiki/Chloroquine ## Full text of document (backup) An Effective Treatment for Coronavirus (COVID-19)_ An Effective Treatment for Coronavirus (COVID-19) Presented by: James M. Todaro, MD (Columbia MD, jtodaro2@gmail.com) and Gregory J. Rigano, Esq. (grigano1@jhu.edu) In consultation with Stanford University School of Medicine, UAB School of Medicine and National Academy of Sciences researchers. March 13, 2020 SPANISH: https://docs.google.com/document/d/e/2PACX-1vR1adodKPhWalV9djnerI2x_v1LGgGyhZZxpl0O5r-ZNyDdagqFq1rTCxXBqaeicfxgvypDOqKCZVyV/pub Translation by: Celia Martínez-Aceves (Yale B.S. Candidate 2021; celia.martinez-aceves@yale.edu), Martín Martínez (MIT B.S. 2017 ; martin.martinez.mit@gmail.com) ITALIAN: https://docs.google.com/document/d/e/2PACX-1vSjPNh_WX6FXUIE3OaA3ScsW7yIH3-SpZyYzElNQUNuJvDmD9eFzM29mVXeaYRY-rjGv52wkrZNa7tb/pub Translation by: Google Translate and edited by Ross Shulman, Cornell University MS '20 ross.shulman@gmail.com Summary Recent guidelines from South Korea and China report that chloroquine is an effective antiviral therapeutic treatment against Coronavirus Disease 2019. Use of chloroquine (tablets) is showing favorable outcomes in humans infected with Coronavirus including faster time to recovery and shorter hospital stay. US CDC research shows that chloroquine also has strong potential as a prophylactic (preventative) measure against coronavirus in the lab, while we wait for a vaccine to be developed. Chloroquine is an inexpensive, globally available drug that has been in widespread human use since 1945 against malaria, autoimmune and various other conditions.
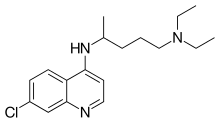
Chloroquine: C18H26ClN3 Background The U.S. CDC and World Health Organization have not published treatment measures against Coronavirus disease 2019 (“COVID-19”). Medical centers are starting to have issues with traditional protocols. Treatments, and ideally a preventative measure, are needed. South Korea and China have had significantly more exposure and time to analyze diagnostic, treatment and preventative options. The U.S., Europe and the rest of the world can learn from their experience. According to former FDA commissioner, board member of Pfizer and Illumina, Scott Gotlieb MD, the world can learn the most about COVID-19 by paying closest attention to the response of countries that have had significant exposure to COVID-19 before the U.S. and Europe.[1] As per the U.S. CDC, “Chloroquine (also known as chloroquine phosphate) is an antimalarial medicine… Chloroquine is available in the United States by prescription only… Chloroquine can be prescribed for either prevention or treatment of malaria. Chloroquine can be prescribed to adults and children of all ages. It can also be safely taken by pregnant women and nursing mothers.”[2] CDC research also shows that “chloroquine can affect virus infection in many ways, and the antiviral effect depends in part on the extent to which the virus utilizes endosomes for entry. Chloroquine has been widely used to treat human diseases, such as malaria, amoebiosis, HIV, and autoimmune diseases, without significant detrimental side effects.”[3] The treatment guidelines of both South Korea and China against COVID-19 are generally consistent, outlining chloroquine as an effective treatment. Specifically, according to the Korea Biomedical Review, in February 2020 in South Korea, the COVID-19 Central Clinical Task Force, composed of physicians and experts treating patients agreed upon treatment principles for patients with COVID-19.[4] In China, the General Office of the National Health Commission, General Office of the State Administration of Traditional Chinese Medicine as well as a Multi-Center Collaborative Group of Guangdong Provincial Department of Science and Technology and Guangdong Provincial Health Comp and the China National Center for Biotechnology Development have established effective treatment measures based on human studies.[5] According to their research (reported in Clinical Trials Arena), “Data from the drug’s [chloroquine] studies showed ‘certain curative effect’ with ‘fairly good efficacy’ … patients treated with chloroquine demonstrated a better drop in fever, improvement of lung CT images, and required a shorter time to recover compared to parallel groups. The percentage of patients with negative viral nucleic acid tests was also higher with the anti-malarial drug… Chloroquine has so far shown no obvious serious adverse reactions in more than 100 participants in the trials… Chloroquine was selected after several screening rounds of thousands of existing drugs. Chloroquine is undergoing further trials in more than ten hospitals in Beijing, Guangdong province and Hunnan province.”[6] Treatment Guidelines from South Korea[7] According to the Korea Biomedical Review, the South Korean COVID-19 Central Clinical Task Force guidelines are as follows: 1. If patients are young, healthy, and have mild symptoms without underlying conditions, doctors can observe them without antiviral treatment; 2. If more than 10 days have passed since the onset of the illness and the symptoms are mild, physicians do not have to start an antiviral medication; 3. However, if patients are old or have underlying conditions with serious symptoms, physicians should consider an antiviral treatment. If they decide to use the antiviral therapy, they should start the administration as soon as possible: … chloroquine 500mg orally per day. 4. As chloroquine is not available in Korea, doctors could consider hydroxychloroquine 400mg orally per day (Hydroxychloroquine is an analog of chloroquine used against malaria, autoimmune disorders, etc. It is widely available as well). 5. The treatment is suitable for 7 - 10 days, which can be shortened or extended depending on clinical progress. Notably, the guidelines mention other antivirals as further lines of defense, including anti-HIV drugs. Treatment Guidelines from China[8] According to China’s Novel Coronavirus Pneumonia Diagnosis and Treatment Plan, 7th Edition, the treatment guidelines are as follows: 1. Treatment for mild cases includes bed rest, supportive treatments, and maintenance of caloric intake. Pay attention to fluid and electrolyte balance and maintain homeostasis. Closely monitor the patient's vitals and oxygen saturation. 2. As indicated by clinical presentations, monitor the hematology panel, routine urinalysis, CRP, biochemistry (liver enzymes, cardiac enzymes, kidney function), coagulation, arterial blood gas analysis, chest radiography, and so on. Cytokines can be tested, if possible. 3. Administer effective oxygenation measures promptly, including nasal catheter, oxygen mask, and high flow nasal cannula. If conditions allow, a hydrogen-oxygen gas mix (H2/O2: 66.6%/33.3%) may be used for breathing. 4. Antiviral therapies: ... chloroquine phosphate (adult 18-65 years old weighing more than 50kg: 500mg twice daily for 7 days; bodyweight less than 50kg: 500mg twice daily for day 1 and 2, 500mg once daily for day 3 through 7) … Additionally, the Guangdong Provincial Department of Science and Technology and the Guangdong Provincial Health and Health Commission issued a report stating “Expert consensus on chloroquine phosphate for new coronavirus pneumonia: … clinical research results show that chloroquine improves the success rate of treatment and shortens the length of patient’s hospital stay.”[9] The report further goes on to cite research from the US CDC from 2005 as well as research from the University of Leuven University in Belgium regarding chloroquine’s effectiveness against SARS coronavirus at the cellular level.[10] Like the South Korean guidelines, notably, other antivirals (e.g. anti-HIV drugs) are listed as further lines of defense. The most research thus far has been around chloroquine. Chloroquine as a prophylactic (preventative) measure against COVID-19[11] According to research by the US CDC, chloroquine has strong antiviral effects on SARS coronavirus, both prophylactically and therapeutically. SARS coronavirus has significant similarities to COVID-19. Specifically, the CDC research was completed in primate cells using chloroquine’s well known function of elevating endosomal pH. The results show that “We have identified chloroquine as an effective antiviral agent for SARS-CoV in cell culture conditions, as evidenced by its inhibitory effect when the drug was added prior to infection or after the initiation and establishment of infection. The fact that chloroquine exerts an antiviral effect during pre- and post-infection conditions suggest that it is likely to have both prophylactic and therapeutic advantages.” The study shows that chloroquine is effective in preventing SARS-CoV infection in cell culture if the drug is added to the cells 24 h prior to infection.

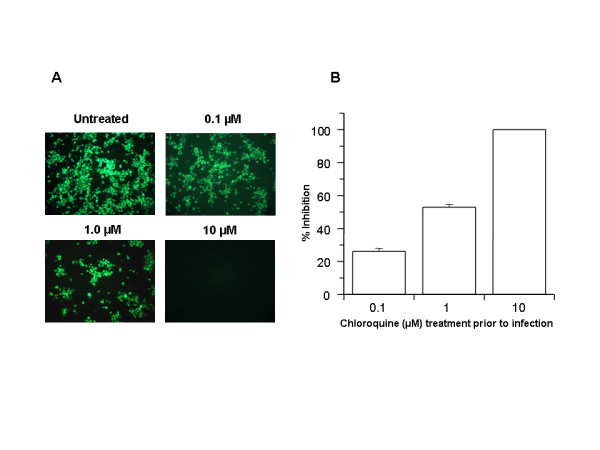
FIGURE 1 Prophylactic effect of chloroquine. Vero E6 cells pre-treated with chloroquine for 20 hrs. Chloroquine-containing media were removed and the cells were washed with phosphate buffered saline before they were infected with SARS-CoV (0.5 multiplicity of infection) for 1 h in the absence of chloroquine. Virus was then removed and the cells were maintained in Opti-MEM (Invitrogen) for 16–18 h in the absence of chloroquine. SARS-CoV antigens were stained with virus-specific HMAF, followed by FITC-conjugated secondary antibodies. (A) The concentration of chloroquine used is indicated on the top of each panel. (B) SARS-CoV antigen-positive cells at three random locations were captured by using a digital camera, the number of antigen-positive cells was determined, and the average inhibition was calculated. Percent inhibition was obtained by considering the untreated control as 0% inhibition. The vertical bars represent the range of SEM. In the case of chloroquine treatment prior to infection, the impairment of terminal glycosylation of ACE2 may result in reduced binding affinities between ACE2 and SARS-CoV spike protein and negatively influence the initiation of SARS-CoV infection. The cell surface expression of under-glycosylated ACE2 and its poor affinity to SARS-CoV spike protein may be the primary mechanism by which infection is prevented by drug pretreatment of cells prior to infection. In addition, the study also shows that chloroquine was very effective even when the drug was added 3–5 h after infection, suggesting an antiviral effect even after the establishment of infection.
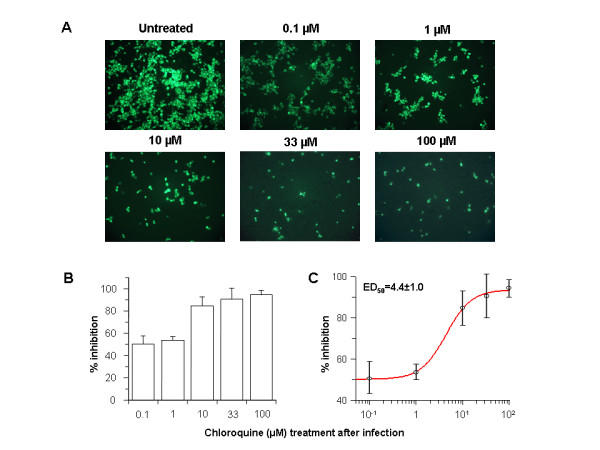
Figure 2 Post-infection chloroquine treatment reduces SARS-CoV infection and spread. Vero E6 cells were seeded and infected as described for Fig. 1 except that chloroquine was added only after virus adsorption. Cells were maintained in Opti-MEM (Invitrogen) containing chloroquine for 16–18 h, after which they were processed for immunofluorescence. (A) The concentration of chloroquine is indicated on the top. (B) Percent inhibition and SEM were calculated as in Fig. 1B. (C) The effective dose (ED50) was calculated using commercially available software (Grafit, version 4, Erithacus Software). When chloroquine is added after infection, it can rapidly raise the pH and subvert on-going fusion events between virus and endosomes, thus inhibiting the infection. When added after the initiation of infection, it likely affects the endosome-mediated fusion, subsequent virus replication, or assembly and release. Specifically, rapid elevation of endosomal pH and abrogation of virus-endosome fusion may be the primary mechanism by which virus infection is prevented under post-treatment conditions. The US CDC study goes on to conclude that: “The infectivity of coronaviruses other than SARS-CoV are also affected by chloroquine, as exemplified by the human CoV-229E [15]. The inhibitory effects observed on SARS-CoV infectivity and cell spread occurred in the presence of 1–10 μM chloroquine, which are plasma concentrations achievable during the prophylaxis and treatment of malaria (varying from 1.6–12.5 μM) [26] and hence are well tolerated by patients. Chloroquine, a relatively safe, effective and cheap drug used for treating many human diseases including malaria, amoebiasis and human immunodeficiency virus is effective in inhibiting the infection and spread of SARS CoV in cell culture.” COVID-19 and Chloroquine: Mechanisms of Action[12] COVID-19 in a single stranded, positive strain RNA virus with a protein shell and membrane. The genome is of the same sense of the mRNA. It goes through a lifecycle where incoming viral COVID genome has to become double stranded RNA and the new strand becomes the new strand for the new mRNA. There are significant similarities between COVID-19 and SARS coronavirus. Both COVID-19 and SARS-like coronaviruses have machinery for regulating their own replication and production of their proteins. Coronavirus depends on the breakdown of macromolecules such as proteins. Specifically, the virus depends on turning over the host proteins to trigger response for available building blocks to make their own proteins or nucleic acids. They break down due to low PH catalyzed by hydrolysis. Additionally, coronaviruses have non-structural proteins that are not part of the capsid (protein shell of the virus). These non-structural proteins are regulatory proteins that take over the host cell and suppress the immune system of the host (similar to HIV). Coronavirus can create growth factor like mechanisms (e.g. cytokines) to optimize the growth environment in the cell to favor it. It is this part of the coronavirus’ replicative path that chloroquine inhibits. Notably, because of its nitrogen structure, chloroquine has the unique ability to get into cells and cross endosomal membranes. Once inside, nitrogens in chloroquine (and quinines in general) prevent acidification by absorbing a high amount of hydrogens that simply then interact with nitrogen and then chloroquine becomes positively charged - an ionic interaction which makes it harder for the endosome to become acidified. The result is a buffer that holds it at the higher pH and prevents it from becoming acidic enough to be functional. To summarize, because chloroquine has a multitude of extra nitrogens, once it crosses the membrane and enters an organelle, the organelle is prevented from reaching a lower pH. The organelle’s enzymes cannot work because the donor group will be a hydrogen ion, disabling the hydrolysis required for coronavirus replication. This means that all kinds of events in the cell are incapable of performing optimally, including viral replication. Chloroquine’s entrance into the organelle likely constipates the whole system. An analogy is that the virus is like a garbage facility which has to break down and burn up the garbage and if it cannot, the garbage piles up and the city becomes paralyzed. This is likely the case for any virus, cancer cells or any other condition that is dependent on turning over the worn out or incorrectly synthesized proteins. The UK has banned the export of Chloroquine[13] As of February 26, 2020, the UK government has added chloroquine to the list of medicines that cannot be parallel exported from the UK. Chloroquine was never on this list before. This likely happened because of the growing body of evidence of chloroquine’s effectiveness against coronavirus. China prioritizes internal use of Active Pharmaceutical Ingredients (APIs) including Chloroquine[14] In early February, Chongqing Kangle Pharmaceutical was requested by the Ministry of Industry and Information Technology, Consumption Division to promptly increase the manufacturing and production of the active pharmaceutical ingredients chloroquine phosphate despite slowed production during the Chinese New Year. Key Risks and Tradeoffs There has been massive de-stabilization of society due to COVID-19. Mutations[15] RNA viruses are subject to fairly high mutation rates as RNA based genomes do not copy themselves faithfully, thereby accumulating mutations quickly which can lead to failure of the virus (analogy: unaudited software code will often eventually fail due to a critical error) or can lead to a stronger mutation - which is likely what has happened in 2020 (when coronavirus “jumped” from animal to human; it is doubtful that this has occurred because of the use of chloroquine) as we have have two forms of COVID-19 (“more aggressive” and “less aggressive”). If the replication quality of RNA virus like coronavirus can be destabilized this will likely cause it to self destruct, but there is always the risk that the virus mutates to become more aggressive. Treating COVID-19 with chloroquine, as is being done in South Korea and China does have the potential to lead to a mutation. The mutation can either be beneficial or harmful to humans. In this particular case, chloroquine is likely being used to destabilize the replication quality of COVID-19, providing significant potential for COVID-19 to self-destruct, which would likely bide more time for health systems worldwide to increase capacity and equipment as well as allow time for the public release of a vaccine. All precaution must be taken into account for the risk of escape where COVID-19 comes out stronger. Manufacturing Chloroquine and its analogs has been manufactured and distributed at global scale since approximately 1945. While there has recently been a shortage of N95 protective masks, medical systems can adjust and dramatically increase the supply of chloroquine in the world. Chloroquine tablets and intravenous formulations are generic and easy to produce. Safety[16] Chloroquine is a prescription drug. It can have side effects and has contraindications. One often cited side effect is chloroquine retinopathy, which can result in permanent vision loss after high cumulative doses of chloroquine. However, retinal damage is extremely rare in patients with a total dosage under 400g (dosage level only reached after years of treatment). Medical professionals must be consulted before use of chloroquine. Chloroquine tablets are readily available in the U.S. and have never been removed from the market. Intravenous chloroquine was taken off the market in the USA pre-2000 because of the absence of acute malarial infections in the USA - there was no use for the intravenous form. It can easily be brought back to the market. Formulation Optimizations[17] Tablet vs. Intravenous Currently chloroquine is most widely administered in tablet form (chloroquine phosphate. While readily available, the issue is that when the tablet is ingested, it must be processed through the stomach and be taken up by the small intestine, for which then it enters the blood and subsequently the respiratory system. Because of the metabolism, this takes time and there is a loss of chloroquine delivery to the respiratory system (where COVID-19 replicates). When chloroquine is used intravenously against malaria (chloroquine hydrochloride), it is being mainlined directly into the blood stream so that it is distributing around the body within seconds, likely encountering the virus faster and at a higher concentration in the respiratory system. Intravenous formulations are readily available and should be studied accordingly. Further research should be carried out using chloroquine in nanoparticles and various fast, slow and sustained released formulations, as well as combinations of chloroquine and other molecules. Repurposing other FDA approved drugs As per Steve Schow PhD, Professor of Chemical and Systems Biology at Stanford University School of Medicine and Lead Advisor to Stanford’s SPARK Translational Research Program: “There are a number of related isoquinoline and quinoline drug family members who might exhibit the same general acid neutralizing effects. In addition certain antidepressants and antipsychotic drugs are known to accumulate in lysosomes via this acid-base process and might be effective here if the doses needed aren’t too high.”[18] New Molecular Entity: Chloroquine analogs with more nitrogens The nitrogens in chloroquine and quinines in general prevent acidification by absorbing a high amount of hydrogens that then interact with nitrogen, and,in turn, transfer a positive charge to chloroquine. This ionic interaction makes it harder and harder for the endosome to become acidified, therefore disrupting viral replication. If more nitrogens are added, either by making extra branches of ionizable nitrogens or lengthening one of the chains by putting extra carbons and other nitrogens around it, this may have even greater effect. The key issue will be whether there is a heavy change in bioavailability - will the new molecule be able to enter the cell and reach the right place with similar efficiency. Conclusion Chloroquine can both prevent and treat malaria. Chloroquine can both prevent and treat coronavirus in primate cells (Figure 1 and Figure 2). According to South Korean and China human treatment guidelines, chloroquine is effective in treating COVID-19. Given chloroquine’s human safety profile and existence, it can be implemented today in the U.S., Europe and the rest of the world. Medical doctors may be reluctant to prescribe chloroquine to treat COVID-19 since it is not FDA approved for this use. The United States of America and other countries should immediately authorize and indemnify medical doctors for prescribing chloroquine to treat COVID-19. We must explore whether chloroquine can safely serve as a preventative measure prior to infection of COVID-19 to stop further spread of this highly contagious virus. More Sources Griffero-Diaz's F. , Hoschander SA , Brojatsch J .Endocytosis IS A Critical entry in STEP B of subgroup Avian leukosis viruses[J].J Virology,2003,76(24):12866-12876.The DOI: 10.1128 / jvi.76.24. 12866-12876.2002 . Rodrigo D , Luiza H , Paula P , et al .Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models[J].Viruses,2016,8(12):322-.DOI: 10.3390 / v8120322 . Zhang S , Yi C , of Li C , et Al .Chloroquine inhibits the endosomal Viral an RNA Release and autophagy in-dependent Viral Replication and Effectively Prevents CARE OF to Fetal Transmission of Zika Virus. [J] Antiviral Res.2019;169:104 547. The DOI: 10.1016 /j.antiviral.2019.104547 Kono M , Tatsumi K , Imai AM , et al .Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK[J].Antiviral Res,2008,77(2):150-152.DOI: 10.1016 / j.antiviral.2007.10.011 . Didier Raoult, et. al. , Chloroquine and hydroxychloroquine as available weapons to fight COVID-19 International Journal of Antimicrobial Agents Available online 4 March 2020, https://www.sciencedirect.com/science/article/pii/S0924857920300820?via%3Dihub#! Next Steps from the Community Disseminate this publication amongst the medical community. Get more feedback. Send this publication to your scientific contacts in South Korea and China - lets get more data, details, etc. Science never ends. Translate this paper into all languages. Explore all options for use of chloroquine against any medical condition that depends on the turnover of worn out or incorrectly synthesized proteins. Acknowledgements Special thanks to Stanford University School of Medicine, SPARK Translational Research Program, Steve Schow, PhD, The Lab of Louise T. Chow, PhD and Thomas R. Broker, PhD, Bruce Bloom DDS, JD of HealX and Adrian Bye. License Due to urgency, certain parts of this publication are taken directly from their attributed source. Cite them accordingly. In all other circumstances, the GNU General Public License v3.0 applies. Disclaimer This white paper is for information purposes only. The authors and or its affiliates does not guarantee the accuracy of or the conclusions reached in this white paper, and this white paper is provided “as is”. The authors and or its affiliates not make and expressly disclaims all representations and warranties, express, implied, statutory or otherwise, whatsoever, including, but not limited to: (i) warranties of merchantability, fitness for a particular purpose, suitability, usage, title or noninfringement; (ii) that the contents of this white paper are free from error; and (iii) that such contents will not infringe third-party rights. The authors and or its affiliates shall have no liability for damages of any kind arising out of the use, reference to, or reliance on this white paper or any of the content contained herein, even if advised of the possibility of such damages. In no event will the authors and or its affiliates be liable to any person or entity for any damages, losses, liabilities, costs or expenses of any kind, whether direct or indirect, consequential, compensatory, incidental, actual, exemplary, punitive or special for the use of, reference to, or reliance on this white paper or any of the content contained herein, including, without limitation, any loss of business, revenues, profits, data, use, goodwill or other intangible losses. All translations are done voluntarily by third-parties for which the authors have no affiliation - we do not attest to their accuracy. Informational Purposes Only [1] https://www.cnbc.com/video/2020/03/02/coronavirus-testing-emergency-room-doctor-cdc-department-health-squawk-box.html [2] https://www.cdc.gov/malaria/resources/pdf/fsp/drugs/Chloroquine.pdf [3] Vincent, Martin J et al. “Chloroquine is a potent inhibitor of SARS coronavirus infection and spread.” Virology journal vol. 2 69. 22 Aug. 2005, doi:10.1186/1743-422X-2-69 , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1232869/#B15. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [4] http://www.koreabiomed.com/news/articleView.html?idxno=7428 [5] https://www.ncbi.nlm.nih.gov/pubmed/32075365/ ; http://www.nhc.gov.cn/yzygj/s7653p/202002/0293d017621941f6b2a4890035243730.shtml translated as https://www.chinalawtranslate.com/en/chloroquine-phosphate/ ; Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 7th Edition) translated as https://www.chinalawtranslate.com/en/coronavirus-treatment-plan-7/ ; https://www.clinicaltrialsarena.com/news/coronavirus-covid-19-choroquine-data/ . [6] https://www.clinicaltrialsarena.com/news/coronavirus-covid-19-choroquine-data/ . This research must be confirmed and furthermore ruled out that the subjects that had negative viral nucleic acid tests might not have been infected with C-19. [7] http://www.koreabiomed.com/news/articleView.html?idxno=7428 [8] Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Provisional 7th Edition)translated as https://www.chinalawtranslate.com/en/coronavirus-treatment-plan-7/ [9] https://www.ncbi.nlm.nih.gov/pubmed/32075365/ Guangdong Provincial Science and Technology Department and Guangdong Provincial Health and Health Commission's Multicenter Collaboration Group on Chloroquine Phosphate for New Coronavirus Pneumonia. Expert Consensus on Chloroquine Phosphate for New Coronavirus Pneumonia [J / OL]. Chinese Journal of Tuberculosis and Respiratory Medicine, 2020,43 (2020-02-20) .http: //rs.yiigle.com/yufabiao/1182323.htm. [10] US CDC, Vincent MJ , Bergeron E , Benjannet S , et Al .Chloroquine IS A potent inhibitor of SARS coronavirus Infection and Spread of[J].Virology Journal,2005,2(. 1):69.The DOI: 10.1186 / 1743-422X-2-69 . Keyaerts E , Vijgen L , Maes P , et Al .The In Journal Severe acute Inhibition of Respiratory syndrome coronavirus by chloroquine[J].Biochem Biophys Res Communications,2004,323(. 1):0-268.The DOI: 10.1016 / j.bbrc .2004.08.085 . [11] All research from this section is from: US CDC, Vincent MJ , Bergeron E , Benjannet S , et Al .Chloroquine IS A potent inhibitor of SARS coronavirus Infection and Spread of[J].Virology Journal,2005,2(. 1):69.The DOI: 10.1186 / 1743-422X-2-69 [12] All research from this section is from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4147684/ , https://virologyj.biomedcentral.com/articles/10.1186/s12985-019-1182-0#citeas ,https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1232869/#B15 , https://www.nature.com/articles/s41422-020-0282-0 , Thomas R. Broker, PhD, Stanford University School of Medicine, Telephone discussion March 12, 2020 , https://www.sciencealert.com/genetic-analysis-shows-wuhan-coronavirus-is-similar-to-sars . [13] https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/872567/medicines_that_cannot_be_parallel_exported_from_the_uk_13_march_2020.csv/preview [14] http://doc.irasia.com/listco/hk/tfkf/announcement/a224851-e_01312ann_20200203(20200203_1952).pdf [15] All information in this section is from: https://www.sciencealert.com/genetic-analysis-shows-wuhan-coronavirus-is-similar-to-sars , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4147684/ , https://virologyj.biomedcentral.com/articles/10.1186/s12985-019-1182-0#citeas , Thomas R. Broker, PhD, Stanford University School of Medicine, Telephone discussion March 12, 2020. [16] https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/006002s044lbl.pdf , https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=006002 , https://www.cdc.gov/malaria/resources/pdf/fsp/drugs/Chloroquine.pdf [17] See Safety citations. [18] Steve Schow PhD, https://sparkmed.stanford.edu/about-spark/who-we-are/ . Email correspondence March 2020. Published by Google Drive–Report Abuse–Updated automatically every 5 minutes

Originally posted here: https://steemit.com/coronavirus/@darkflame/use-of-chloroquine-tablets-is-showing-favorable-outcomes-in-humans-infected-with-coronavirus




No comments:
Post a Comment